What is the Difference Between Viscose & Polyester?
How much do you know about man-made fibres? You may well be familiar with the names: Nylon, Polyester, Viscose and Rayon. Do you know how they are similar and how they are different? Do you know which works best for you and why?
In this article, we take a closer look at two of the most popular man-made fibres: polyester and viscose. Both these fibres may be man-made, but don't let that fool you, there is a world of difference in how they feel and how they work when worn as clothing.
Disclosure: We use bamboo viscose fabric in our range of men's undershirts.
To keep things simple, we will look at how polyester and viscose are used in everyday clothing, with a particular interest in how they react to water. Then, for those who are interested in science, we will dive down into the atomic and microscopic levels to see what makes polyester and viscose so different.
Polyester
Polyester is a synthetic fibre made from a compound called polyethylene terephthalate (PET for short), an oil-based material, manufactured in a way similar to many plastics. ThoughF it comes in many forms, we will consider it here in the form you'll find most familiar, the material commonly used in sportswear: a fabric made from smooth and long filament fibres.
Polyester is commonly used as a wicking fabric, or as a water-resistant barrier; two very different uses. How can they be so different? It depends on how the polyester filament fibres are woven, or knitted, into a fabric. Of particular importance is the tightness or looseness of the weave.
Loose-weave polyester: great for wicking sweat away
Do you enjoy a sweat-soaked workout in the gym? Most likely your kit is a stretchy jersey made from knitted polyester. In this looser form, polyester makes an excellent moisture-wicking material enabling your sweat to wick away and evaporate in the open air. How does this happen? The critical factor is that water can not enter the polyester fibre; it is actively repelled from the surface (see FACT BOX below - Why Oil and Water Don't Mix). This repulsion of water encourages sweat to migrate along the length of each fibre, working its way through the gaps in the fabric until it reaches the outside of the fabric where it can evaporate. This mechanism also explains why polyester dries quickly. The ability to move moisture around means the water does not hang about, which is why your gym kit the first to dry after being washed. All well and good, but there is one small problem: for some reason, polyester encourages the growth of odour producing bacteria.
Tight-weave polyester: a water barrier
Tightly woven polyester acts as a barrier to water because a tight weave makes any gaps between the threads too small for water droplets to pass - ideal rainproofing. If the weave is sufficiently tight, it will even prevent water vapour from passing; in which case the fabric does not 'breathe'. This property might be perfect for a weather-resistant camera bag, but it's less than ideal for most indoor clothing. Workplace uniforms are sometimes made from 100% polyester fabric in a tight weave because it is cheap, maintains bright colours and is easy to maintain. However, because the fabric does not breathe well, water vapour stays trapped next to the skin, leaving the wearer feeling hot, sticky and uncomfortable. Happily, even cheaper uniforms these days tend to be made from a polyester-cotton blend (known as polycotton) to overcome some of these problems.
Viscose
Viscose works differently to polyester. Viscose is a semi-synthetic fibre made from a compound known as cellulose – a plant-based material. Like polyester, it is also formed in long smooth filament fibres, but that is where the similarities end.
Viscose: great for absorbing sweat
Unlike polyester, viscose is water-absorbing. Each fibre of viscose has tiny spaces running inside called nanopores. Unlike oil-based polyester which repels water, the cellulose-based viscose encourages water to seep into these nanopores so wetting the inside of the fibre itself. It is this affinity to water that makes viscose so good at absorbing water. It also makes the fabric highly breathable, which is essential for comfort. Breathable fabrics remove water vapour (and heat) from your skin, leaving you feeling cool, dry and comfortable. Viscose does this well, which is why it's such an excellent material for underwear.
When it comes to sweat, regular amounts of sweat can be absorbed into the fabric and locked away, preventing sweat marks on your shirt - making it a popular choice for undershirts. It also doesn't suffer the same stinky microbe problem that plagues polyester. It's not clear why that is the case, but a theory is that microbes can't grow on viscose because all the available water is locked away inside the fibre. Bacteria prefer to breed in the tiny droplets of water found on the surface of polyester fibres.
But wait! It's not all rosy for viscose: it has its problems. Overwhelm viscose with too much water (sweat), and it will become saturated and clammy. Moreover, because it likes to absorb water, not repel it, it needs more time to dry after washing.
Summary
Both polyester and viscose are man-made, but oil-based polyester repels water while plant-based viscose attracts and absorbs water.
Polyester fabric is good at wicking water away, making it ideal for the gym. But unfortunately, it may pong after wearing because water droplets on the surface of the fibres make ideal breading conditions for odour producing bacteria.
Viscose has a high affinity for water, so it's excellent for making highly breathable fabrics which absorb water and water vapour, releasing heat from the body. It also seems to prevent microbes from growing, making it less prone to odour. All of this mean viscose is ideal for wearing next to the skin: underwear, socks, undershirts etc.
To sum up: if you want a weather-resistant bag or raincoat, make it out of polyester. By contrast, for comfort, make sure your underwear is made from viscose (or cotton at a push). There is a reason why no-one wears polyester underpants!
Deeper into the Science
The chemical difference between viscose and polyester
If you were to peer inside a single fibre of either viscose or polyester, right down to its atoms, you would see a long chain of a repeating molecule. This process of joining long chains of the same molecule is called polymerisation, and it allows us to make materials with uniform and precise characteristics. That is why both polyester and viscose can be made as long, smooth, silk-like filaments.
However, even though polyester and viscose fibres might look outwardly similar, their characteristics (affinity to water, strength, and so on) is determined by their base material. Polyester is a long chain of repeating PET molecules, whereas viscose is a long chain of cellulose molecules.
PET (polyethylene terephthalate) is made by combining terephthalic acid and ethylene glycol – both of which are products of the petrochemical industry. Cellulose, on the other hand, is a naturally occurring plant material (cotton is 90% cellulose). The cellulose used in viscose is typically extracted from trees, although bamboo is becoming an increasingly popular source because it is fast growing.
Both viscose and polyester start life as a fluid containing a form of the base molecule. This fluid is spun into long, smooth filaments – not unlike silk. It is during the spinning process that the polymerisation (chaining) takes place: the filaments consist of endlessly repeating patterns of their base molecule. These filaments are then made into yarns that are later woven, or knitted, into fabrics.
FACT BOX - Why Oil and Water Don't Mix
Ever wondered why oil and water don't mix? The answer lies at the molecular level. Specifically, it depends on the distribution of the constituent atom's electrons and how freely they can move.
To be, or not to be, polar
Molecules which have more electrons at one end than the other are said to be electrically polarised. The end with more of the elections has a negative charge (electrons are themselves negative); the other end has a positive charge. Water is one such example of a polarised molecule. Non-polar molecules do not have differently charged ends. Here the electrons are distributed evenly. They are also held firmly in place so they can't move around or gather in one place. The result is no charge at all. Oils (and fats in general) are non-polar.
Opposites attract
The old saying is true for charged particles. Polarised molecules find each other attractive. The positively charged end of one molecule will attract the negatively charged end of another molecule. This draws the two molecules together and they 'hang out'. Water is especially good at this - its molecules love to hang out together, forming what are known as week hydrogen bonds. It's this linking together of molecules that gives water some of its physical properties such as: forming large droplets, have a 'skin' on which insects can skate, and for its unusually high boiling point for its molecular weight. In fact, water molecules need some encouragement to break apart and stop hanging out in big clumps (water appears to be quite cliquey). One such way is to introduce another polarised molecule. Because it too has positive and negative ends this new molecule also attracts water molecules which bind around it. The new substance appears to disappear into the water - this is the mechanism of dissolving.
Oil ain't got no attraction
Oil is non-polar; the elections are evenly distributed, so there is no positively or negatively charged end. When oil is mixed with water, the water molecules simply ignore it. There's nothing else to be attracted to, so the water molecules stay clumped together. The oil can't break in as such, so it remains separate to the water. That's why oil and water don't mix. It's also why oil-based products like polyester and other plastics are water-resistant and why they seem to repel water, which tends to form droplets on the surface which can readily roll-off.
So far, so good. But why then do the fabrics of polyester and viscose behave so differently? The answer lies in the balance of electrical charge across the atoms of each base molecule.
In polyester, the electrical charge is distributed evenly across the whole of the PET molecule. In contrast, the electrical charge surface of the viscose cellulose molecule is polarised: one end of the molecule carries a positive charge, and the other end has a negative charge. This uneven distribution of electrical charge makes all the difference to how the cellulose interacts with water. But why is that?
Viscose: water-loving (hydrophilic)
The polarisation of the cellulose molecule makes it attractive to water molecules. Why? Because water is also a polarised molecule. You know that saying, 'opposites attract'? Well, that is what happens when these two molecules get together. The positive end of the water molecule is attracted to the negative end of the cellulose molecule, and vice versa. The cellulose molecule is said to be hydrophilic, or water-loving.
This attraction allows each water molecule to get very close to each cellulose molecule, resulting in a high degree of 'wetting': the water molecules separate from each other and spread out all over the cellulose structure. Here's the crux: inside the viscose fibres are many tiny spaces, sometimes called 'nanopores'. The water molecules fill up all these nanopores inside the fibre. This is how viscose fibres absorb water, and this, in turn, has is an important factor for how the textile feels against your skin.
Polyester: water-repelling (hydrophobic)
Polyester is the opposite of viscose. The PET molecule has an even charge all over (non-polarised), making it water-repelling, or hydrophobic. The lack of positive electrical charge at one end means water molecules have nothing to attach to, apart from each other. Consequently, they stay clumped together in a liquid droplet on the surface of the fibre. At an atomic level, the fibre itself is not 'wetted': there is no bonding between the PET molecules and the water molecules, so the water molecules are not pulled apart from each other - they remain as large droplets. Water droplets are too large to enter the structure of the fibres, so instead, they travel across the surface. This mechanism is what makes polyester a 'wicking' fabric.
This single difference explains why polyester is water repelling and why viscose is water absorbing.
'Wetting' under the microscope
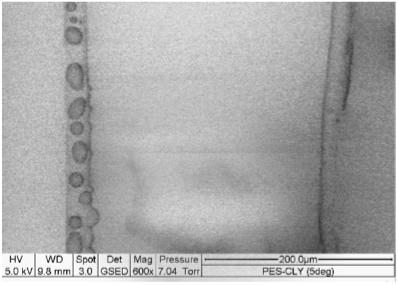
You can see this 'wetting' principle in action below.
The picture shows one Polyester fibre on the left and one Tencel® fibre (Tencel® is a brand of viscose) on the right under a scanning electron microscope. Both fibres are held in the same controlled atmosphere, which is saturated with water; this atmosphere forces the fibres to react to water – just as if you were wearing clothing made from these fibres on a hot and humid day.
Picture credit LENZING AG
On the left, you can see water droplets sitting on the surface of the polyester fibre. On the right there are no such droplets: the Viscose fibre has absorbed the water.
Picture credit LENZING AG
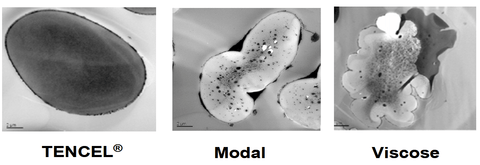
The picture above shows cross-sections of Tencel®, Modal and Viscose fibres – all of which are cellulose-based. In each, the water has been treated so that it shows up as dark areas under the microscope. You can see the dark spots inside the fibre where the water has been absorbed and is filling the fibre's nanopores.
Thank you for reading. If you like to read more about fabrics why not check out the Robert Owen Undershirts research blog.

© 2020 Robert Owen Undershirts





